FDA Approves Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder
The U.S. Food and Drug Administration (FDA) has approved the use of transcranial magnetic stimulation (TMS) as a new treatment option for obsessive-compulsive disorder (OCD). Following successful use in treating depression, the FDA officially expanded the therapy’s approval for OCD patients in August.
OCD is a mental illness characterized by intrusive anxious thoughts (obsessions) and behaviors to reduce the resulting anxiety (compulsions). Approximately 1 percent of the U.S. population lives with the condition, and it can be debilitating. OCD is typically treated with psychiatric medication and therapies such as cognitive behavioral Therapy (CBT) or exposure therapy (EP). However, when these options don’t work, TMS may be a safe and effective alternative treatment.
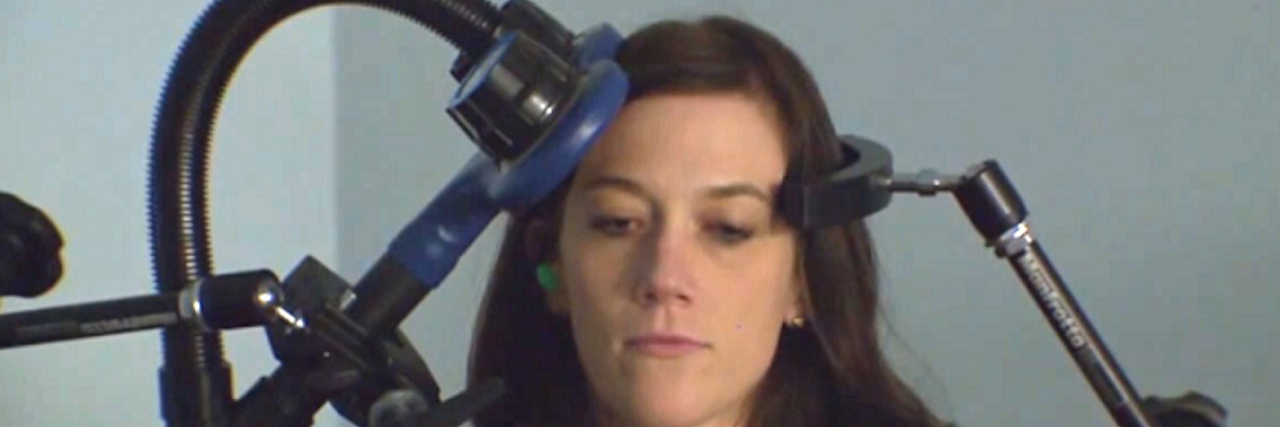
TMS is a magnetic stimulation technique that targets nerves in the brain thought to impact mental illness. The magnetic field, delivered through a machine by a non-invasive coil positioned over the head, stimulates brain cells that regulate mood. Most TMS sessions take 20 to 50 minutes and require no recovery time. The best results typically occur after six weeks of treatment. Though it’s not a permanent fix, patients who receive TMS usually feel better for months up to over a year.
The FDA approved the use of a TMS device made by the company BrainsWay for OCD based on a study of 100 participants diagnosed with OCD at multiple treatment centers. Half of the participants were treated with TMS and the other half a placebo device. Over the course of six weeks, participants were administered five 20-minute sessions of treatment.
In the study, researchers found that TMS successfully reduced OCD symptoms by at least 30 percent — the FDA minimum threshold to display effectiveness — in 38.1 percent of patients after a one-month follow-up. Only 11.1 percent of the control group reported improvement. No major adverse side effects were reported. A similar number of participants who received TMS (37.5 percent) and those who received a placebo (35.3 percent) reported headaches.
However, because of the magnetics involved in TMS, it is not safe to use for those with metallic objects or stimulator devices implanted in or near the head. This includes cochlear implants, deep brain stimulators, vagus nerve stimulators, aneurysm clips or coils, stents, bullet fragments, and even face tattoos with metallic ink. TMS may also increase the risk of seizure, which patients should discuss with their doctor before receiving treatment.
TMS has been approved to treat depression since 2008, especially as an alternative to electroconvulsive therapy (ECT) for those whose illness is treatment resistant. In 2013, the FDA also approved TMS for use for certain migraine conditions. Current studies are still exploring its use for other psychiatric conditions such as bipolar disorder and post-traumatic stress disorder (PTSD).
“Transcranial magnetic stimulation has shown its potential to help patients suffering from depression and headaches,” said Carlos Peña, FDA director of the division of neurological and physical medicine devices, in a statement. “Patients with OCD who have not responded to traditional treatments now have another option.”
Header image via Health.mil/Air Force Staff Sgt. Chad Usher.